|
Water: The Stuff that Makes
Snowflakes

Adapted from
C.W. Fetter, "Applied Hydrology"
Discussion
Using the overhead transparencies provided in the Snow
Curriculum Kit, the instructor introduces the class to the
basic ideas of the hydrologic cycle, water chemistry,
crystal formation, and how a snowflake is formed. There
are many ideas introduced verbally, visually, and physically
in this section. It lays the ground work for the entire
program. Students are encouraged to fill in the blank arrows
on page 2 of the Snow Journal and begin working on their
glossary terms during the discussion. Throughout the program
students will follow along in the snow journals, recording
key terms and ideas.
One of the key concepts is that the planet's water moves
in a cycle and that the total amount of water on the earth
stays fairly constant. This idea that almost all water is
recycled has some interesting ramifications. Ask the
students what they think some of the implications are, and
listen for some wonderful, intuitive answers!
A good tool for teaching the hydrologic cycle is to ask
the students to name a small local creek, and try to trace
the water through its cycle using the local example. In
eastern Denali National Park, the local weather pattern
mostly originates as moisture that has evaporated
from ocean water in the Gulf of Alaska. The moisture
condenses into clouds and typically moves inland and
north. Rain and snow, precipitation, fall when warm
moist air condenses as it encounters the Alaska Range.
Of the resulting runoff, some infiltrates
through to the ground water table, some is actually
trapped on the surface by the frozen permafrost layer below,
and some flows into the creeks as runoff. One creek,
located in eastern Denali Park, Riley Creek, drains the high
mountainous regions. Riley Creek flows into the Nenana
River, which flows into the Tanana, which flows into the
Yukon, which eventually flows into the Bering Sea. There the
water becomes a source for more evaporation and the
resulting cloud formation, and the hydrologic cycle
continues. Attaching this scientific concept to a concrete
local example makes it not only understandable, but real to
the students.
INSTRUCTOR
MATERIALS:
Overhead Transparencies
Overhead Projector
Overhead Pens
Snow Journals
|
|
Chapter
Science
Content
Standard
A - 4, 15
Math
Content
Standard
D 3
English
Language
Arts
Content
Standard
B - 1
Reading
Performance
Standard
age 11-14
1, 3
age 15-18
1, 2, 4

Snow Journal
pg. 2
This journal icon indicates where students use the Snow
Journals in conjunction with the lesson or activity
|
|
English /
Language
Arts
Content
Standard
B - 3
Science
Content
Standards
A - 1, 2 |
How Water
Behaves
|
All matter, including water, is made up of
molecules. Molecules are stable units composed of
smaller particles called atoms. They are the
smallest units of matter that still retain the
characteristics
of that material.
Although invisible to the eye, molecules are
always moving. The energy content, or amount of
motion of the molecules determines the state of
matter (solid, liquid, or gas.)
Matter does not always have to change phases in
sequence. Sublimation is the process where
water, depending upon conditions, can change from
solid to gas or gas to solid without ever passing
through the liquid state.
|
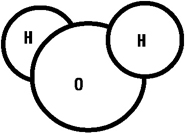
A water molecule. The 120 degree angle of
the two hydrogen atoms flanking the oxygen atom is
the secret to the symmetry of the water crystal
lattice.
|
Activity: Phase Change
Exercise
- Have the entire class stand up, teacher too!
(Shake off those cobwebs). First wiggle and move
as much as possible while still keeping your
feet firmly planted. This motion represents the
molecular vibration of a typical solid.
- Next let's increase the energy, imagine a
bit of heat was added. Now everyone gets to move
as much as possible, but can only move one foot
at a time. This motion represents the liquid
phase, note that the individual molecules are
occupying more space and the room suddenly seems
more crowded.
- In the gas phase simulation, students can
move freely and each individual is encouraged to
move the entire length of the room. Caution! The
gas phase can be a bit unrulely if you have an
especially rambunctious group.
- It also fun to try sublimation where
the students imitate the motion of gas
molecules, and then quickly switch to solid
molecule motion without the intermediate liquid
phase. Remember sublimation can go in both
directions!
This is a good exercise to introduce the concept
of density. Intuitively it is obvious that the gas
is far less dense, intuitively lighter per unit
area.
Most matter expands and becomes less dense as it
changes phases from solid to liquid to gas.
|
|
Water is Different! Freezing
Water and Crystal Formation
|
It is probably not an exaggeration to state that
much of life on the planet as we know it is due to
the unique quality of water becoming less dense as
it freezes.
Does ice sink or float?
It floats, of course, because it is less dense
than water. This phenomenon allows lakes and rivers
to freeze from the top, protecting vital life
processes in the water beneath the ice.
The unique floating quality of ice is due to the
way the molecules arrange themselves as water
crystals are formed in the freezing process. The
arrangement of water molecules is only
semi-structured in the liquidstate. When water
freezes, these molecules assume an orderly
arrangement with fixed positions for the oxygen
atoms. The hydrogen atoms provide the bonds which
hold together this structure called the crystal
lattice. The shape, relative size, and the
electrical charges of the constituent atoms
determine that water molecules will crystallize
during the freezing process into regularly
occurring six sided patterns. The structure of ice
crystals is responsible for the endlessly beautiful
six-sided shape of snowflakes.

|

This diagram shows the crystal lattice of
ice next to free-moving water with a transition
zone of slush. Water's unusual propensity to be
less dense as a solid than as a liquid reflects the
fact that hydrogen atoms shift slightly in angle as
ice crystals form, forcing an increase in
volume.
From "Everyday Science Explained" by Curt
Suplee
|
|
Activity: How Dense is
Snow?
Materials:
Quart-sized jars
Rulers
Tape
Pencil and Markers
Collect snow and fill a jar calibrated from 1
-100. Have students mark their initials on the jar
where they predict the water line will be after
thawing. This can be calculated into a percentage,
and used to introduce the idea of density as
it relates to snow. For example if the water line
is at 22 after the snow has melted, the density
would be 22/100 or 0.22 or 22%. Water is the
standard for density at a value of 1 kg/liter.
The snow of interior Alaska
typically measures in at a density of 0.18 to
0.24 kg/liter, or 18% -24% water content.
|
|
Numeration
Performance
Standard
age 11-14
4
Math
Content
Standard
A -1
|
|
Science
Content
Standards
A 1, 2 |
The Life and Times of a
Snowflake
All precipitation starts as water vapor in the
atmosphere. This water vapor is a product of evaporation and
condensation as previously discussed in the hydrologic cycle
section. As water vapor rises, it usually experiences
cooling. Some of this water vapor will condense into clouds
composed of either ice crystals or water droplets that
remain liquid although the air temperature is below
freezing. In some casesthis supercooled water can
remain liquid in temperatures down to minus 40° Fahrenheit.
Snow crystals begin as minute ice particles which have
formed around condensation nuclei in the atmosphere.
These nuclei are essential to the formation of snow crystals
because smooth sided water molecules will slip by one
another unless there is a solid, rough, surface to hold them
together. There is an abundance of dust in the atmosphere,
but only certain kinds of material attract water vapor.
These nuclei may consist of dust particles or even minute
particles of sea salt. Forest fires, smokestacks, automobile
exhaust, volcanic eruptions, and even barbeques emit
particles that will anchor water molecules to their
surfaces.
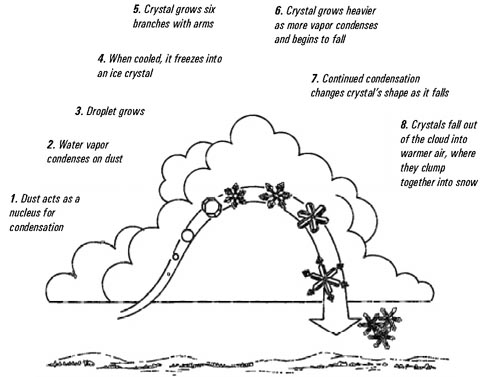
from "Discover Nature in Winter" by Elizabeth
Lawlor
|
|
Snowflakes are made up of very complex ice crystals. One
ice crystal is made up of one quintillion water molecules.
The classic snowflake is a little star of great beauty. The
key to the regularly repeating six sided patterns lies in
the atomic structure of water, the H2O molecule.
Recall the arrangement of the atoms of a water molecule,
with two hydrogen stuck onto a big oxygen like ears on
Mickey Mouse's head. The secret of the snowflake lies in the
angle created by the hydrogen atoms at the center of the
oxygen atom. It is 120 degrees. As a consequence of this
structure, ice forms a stiff six-sided lattice. A crystal,
enlarged by the condensation of water on its surface,
preserves its original symmetry. The famous mathematician
Descartes was the first to record the observation that the
snowflake branches grow only in a direction parallel to the
adjacent arms, always at 60 degrees to their stems. Beside
the feathery stellar shapes, there are needles, columns,
capped columns, and many other shapes.

from "Discover Nature in Winter" by Elizabeth
Lawlor
The particular shape of a snowflake is determined
by the many types of atmospheric conditions it encounters on
its journey from the clouds to the ground. The predominant
factors are temperature and vapor content. Because specific
environmental conditions vary greatly, it is rare that two
snow flakes will take the exact same journey; and so they
say, "No two snowflakes are alike."
|
Science
Content
Standards
A-1, 2, 4 |
A Picture is Worth a Thousand
Words
This graph reveals how temperature and water vapor determine
the particular type of snow crystal that will be formed.

|
Math
Content
Standards
A - 4, 6
Statistics/
Probability
Performance
Standards
age 11-14
2, 4

Snow Journal
pg. 7
|
Air
Temperature
From: "Winter, An Ecological Handbook" by James
Halfpenny and Roy Ozanne.
Cited from Perla and Martinelli, 1976 & Mango and Lee
1966.
|
Activity: Practice Reading the Graph
- Show students that just one illustration can
represent a wealth of information!
- Finding snowflakes on the graph is a great
exercise in graph reading, an important basic
skill.
- Ask students under what temperature and
water vapor conditions Stellar crystals are
formed.
- Next try Solid Bullets, and then, Needles.
They will soon understand how it works!
- Next ask if it is warm and moist, or warm
and dry what crystals will form.
- How does this graph correlate with the
Freshly Fallen Snow Chart in the Snow Journal on
page 6?
|
|
TERMS:
Atom: The smallest possible unit of an element. Atoms of
the same or different elements combine to form molecules. Atoms
remain essentially unchanged during chemical reactions.
Condensation: A change from gas to liquid by cooling.
Condensation Nuclei: Particles of dust that act as a
nucleus, an essential component in the crystallization of water to a
solid.
Crystal: A homogenous solid formed by a repeating 3-D
pattern of atoms or molecules. The orderly arrangement forms a
pattern called a crystal lattice.
Density: The degree to which the atoms of a substance are
packed. Density is the measure of mass per unit of volume. Density
= Mass/ Volume
Evaporation: A change from liquid to gas by heating.
Gas: State of matter having no definite shape or size.
Ground Water: Water moving through permeable subsurface
rock.
Ice Crystal: Water in its solid crystalline state forms a
regularly occurring sixsided lattice of H2O molecules. The
arrangement of the atoms and electrical charges determine the lattice
pattern.
Infiltration: The process of water filtering into the
earth's surface.
Liquid: State of matter having a definite size, but not
shape.
Molecule: Chemical units composed of one or more atoms.
Molecules are smallest particle of a substance having all the
properties of that substance.
Precipitation: Water that falls as rain or snow.
Solid: State of matter having a definite shape and size.
The normal condition of a solid is a crystalline structure.
Sublimation: The direct passage of a substance from solid
to gas or gas to solid without appearing in the intermediate liquid
state.
Supercool: To cool a liquid below the normal freezing point
without solidification
Surface Runoff: The process of water from precipitation
moving downhill into lakes, streams, and rivers.
Notes:
Observing Snow
Introduction
The Four Corners of Life
Water: the Stuff that Makes Snowflakes
Snow on the Ground Changes Through Time
Exploring Native Snow Terms
Glacier Investigations
Open Note Review
Conclusion
Bibliography & Resources