Steambaths
| Steambaths have been an important part of the lives of most Alaskans
for centuries. Beyond the obvious purpose of being a place to get
clean, they have been the center for decision making and spiritual
functions.
Many good illustrations of science principles are found in the steambath. |
|||
|
EvaporationIt takes heat to evaporate water. In a steambath, our body perspires, attempting to cool by evaporation. As our bodies sacrifice water through the skin, the evaporation of that water cools the body. The perspiration that emits from the skin carries with it toxins and other unclean substances. We are cleaned, not just skin deep, but deeper than skin deep. Increased Circulation Some people lightly whip themselves with small bundles of brush. This stimulates blood circulation that helps bring blood to the surface of the skin. |
||
WoodDry wood is very important for a steambath. Damp wood cannot give enough heat. An old man on the Yukon-Kuskokwim Delta used wood scraps from a construction site. Among the wood scraps were pieces of green, pressure-treated lumber. The old man died from the gasses given off. Pressure-treated wood contains arsenic. |
|||
|
Rocks, Steam and Condensation When water is spilled onto the rocks, the heat in the bath feels more intense. Why? Evaporation of water requires heat. Condensation releases heat. When we spill water on the rocks, heat is taken from the rocks to evaporate the water into steam. When the steam condenses into water on our skin, the latent heat of the steam is released onto our bodies. This is why people put grass in their mouths. The steam condenses in the grass, not the lungs. Kinds of Rocks Most white rocks in Interior Alaska are volcanic (igneous), whereas most of the black ones are sedimentary with considerable water trapped within. Long ago, before 55 gallon drums were available to heat the baths, people heated rocks in a campfire outside of the bath. They moved heated rocks into the bath, removing the ones that had cooled. This tedious method is still used when people are camping far away from home. |
||
Hot Air RisesEveryone who has taken a steambath knows that hot air rises. The higher the individual sits, the higher the temperature. For relief, people often lay as close to the floor as possible. |
|||
Soap Polar Substances Water is a polar substance—each molecule is similar to a magnet. One end of the molecule has a partial positive charge, and the other end has a partial negative charge. The materials that dissolve in water are also polar, like salt. Nonpolar Substances Some things, like grease, don’t dissolve in water. Grease is nonpolar. The molecules are not like magnets, they are balanced. Nonpolar liquids, like oil, will dissolve nonpolar substances, like grease, fat, spruce pitch, and some plastics. Soap Is the Link One end of a soap molecule is polar. The other end is nonpolar. It will dissolve a little in water. It will dissolve greases, fats, and other nonpolar substances. It is a link between water and greasy dirt that water alone cannot remove. Conclusion Showers and bathtubs have replaced the steambath in many situations, but the satisfaction of a good steambath cannot be imitated. Steambaths are not likely to be replaced in time, as they provide an arena for social events as well as a thorough cleaning. |
|||

|
Activities
|
||

|
Student Response
|
||

|
Math
|

 Rocks
are important in a steambath because they have enough mass to hold
heat and keep the temperature in the bath steady. If there were
no rocks, the temperature in the bath would rise and fall quickly.
Rocks
are important in a steambath because they have enough mass to hold
heat and keep the temperature in the bath steady. If there were
no rocks, the temperature in the bath would rise and fall quickly.
 When
I first learned about steambaths, people constantly talked about
using white rocks and warned against using black rocks in the bath.
Further inquiry showed that many black rocks contain enough water
that they will often explode dangerously as the internal water turns
to steam. When rocks explode, they sound like a gun blast and pieces
of rock fly in every direction.
When
I first learned about steambaths, people constantly talked about
using white rocks and warned against using black rocks in the bath.
Further inquiry showed that many black rocks contain enough water
that they will often explode dangerously as the internal water turns
to steam. When rocks explode, they sound like a gun blast and pieces
of rock fly in every direction.
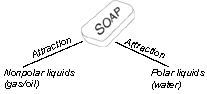 To
understand soap, we must understand polar and nonpolar substances.
To
understand soap, we must understand polar and nonpolar substances.